B
INFINI PREMIUM FILLER BODY
FILLING GEL BASED ON CROSS-LINKED HYALURONIC ACID
INFINI PREMIUM FILLER B BODY is a gel of crosslinked, highly concentrated hyaluronic acid dedicated to restoring or increasing the volume of soft tissues and modeling the body surface. It is perfect for adding youthful volume to the body as well as treating inequalities created after liposuction treatments and volumetric body treatments.
Designed for contouring the silhouette in areas such as buttocks, calves, or hands. INFINI Premium Filler B Body provides a visible volumetric correction by adding long lasting shape and volume to the tissue. INFINI Premium Filler Body unique properties allows practitioners to create enhancements to the tissue achieving client goals. Visibility and durability of the effect depends on the depth of application and the original condition of the skin. INFINI Premium Filler B Body injected into the skin increases the volume of tissue and fills the corrected skin defect.
Cross-linked hyaluronic acid 20mg/ml

INFINI Premium Filler BODY TREATMENT INDICATIONS:
- giving volume to flabby skin
- body shaping and contouring
- buttocks enlargement
- calf shape correction

CLINICAL STUDIES:
Clinical tests conducted by Laboratories and Doctors cooperating with INFINI PREMIUM FILLER confirmed the effectiveness, safety and tolerance of B BODY product in correcting and modelling buttocks. The research group consisted of 50 women aged 24-38 with different body build and lifestyle. The results were assessed by in-vivo examination and ultrasound examination within 6 months after the procedure, using ultrasound equipment: Samsung HS60, linear head 3-14MHz
78% of subjects showed high satisfaction with the improvement of parameters and shape of the corrected area.
90% of respondents appreciated the procedure for its non-invasiveness and short time of postoperative convalescence.
78% of respondents positively assessed the durability and stability of the effect during the study period.
Physicians and patients confirmed the high stability and resistance to movement and deformation of B BODY during the study period. Surgeons and aesthetic medicine specialists appreciated BODY for its safety, satisfactory effects and easy and non-invasive procedure of administration.

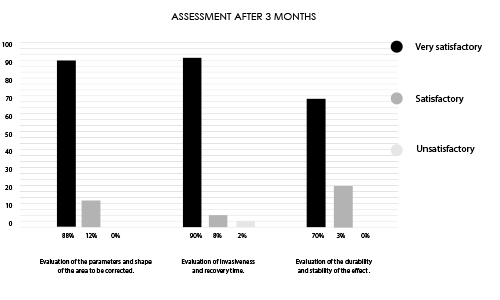
Research conducted on a group of 50 patients undergoing treatment with INFINI PREMIUM FILLER B BODY.
Research group aged 24-38 years of varying body build and lifestyle.
89% of respondents indicated satisfaction with the performed treatment after 3 months.
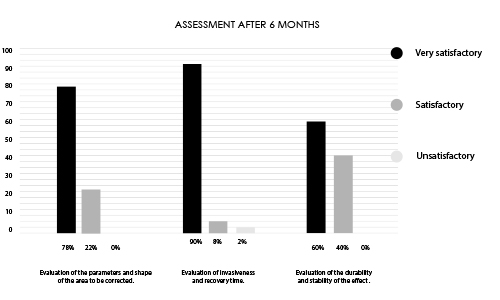
Research conducted on a group of 50 patients undergoing treatment with INFINI PREMIUM FILLER B BODY.
Research group aged 24-38 years of varying body build and lifestyle.
79% of respondents indicated satisfaction with the performed treatment after 6 months.
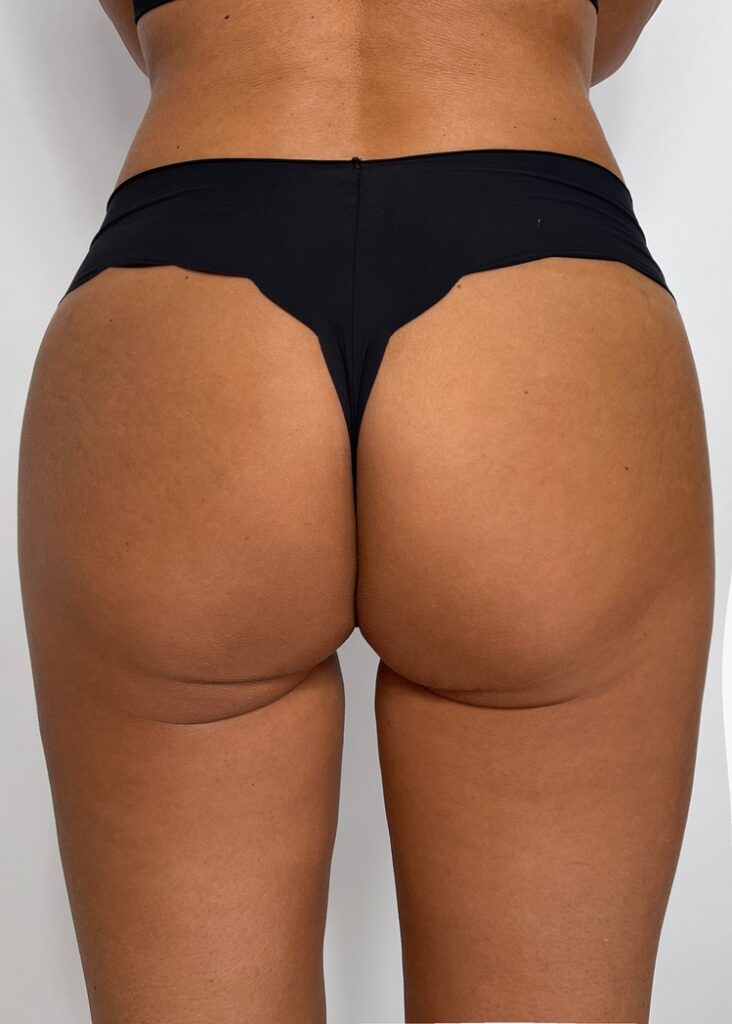
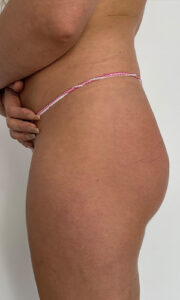
FIRST PATIENT
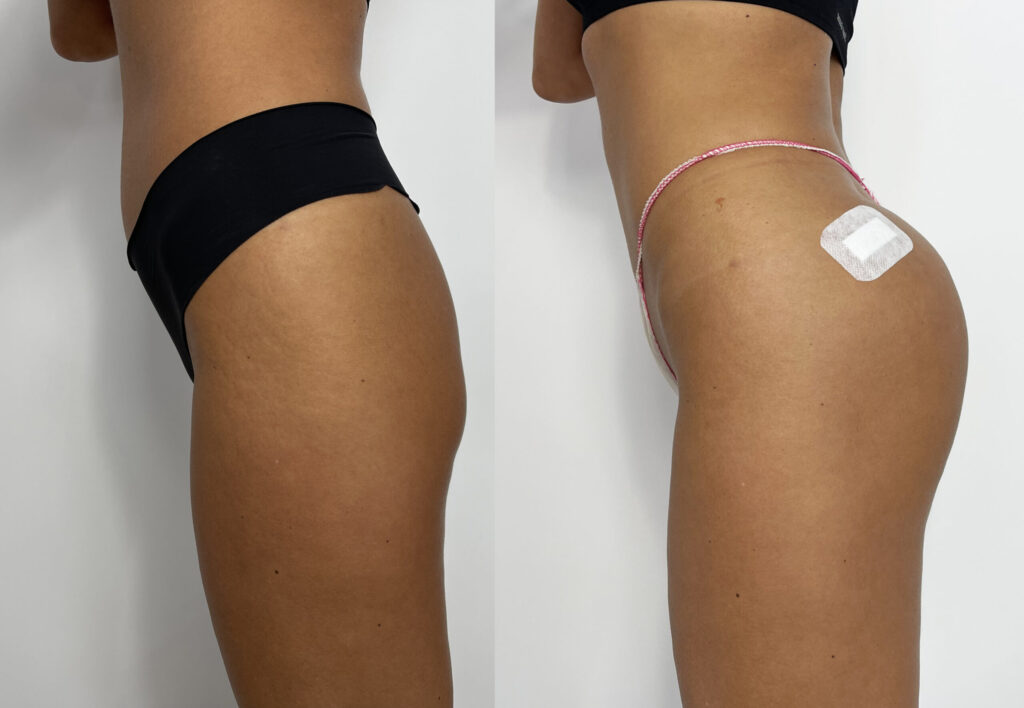

USG BEFORE
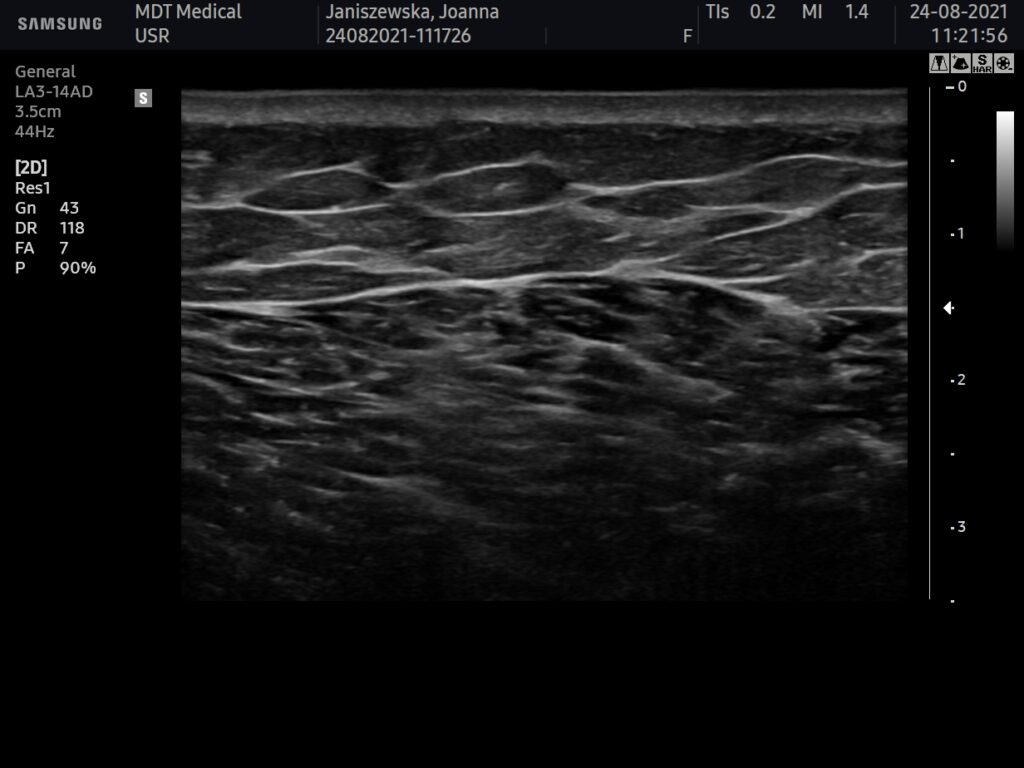
Ultrasound machine: Samsung HS60, linear transducer 3-14MHz
The buttocks area was visualised by ultrasound. No pathology within the skin, subcutaneous tissue and muscles of the buttocks.
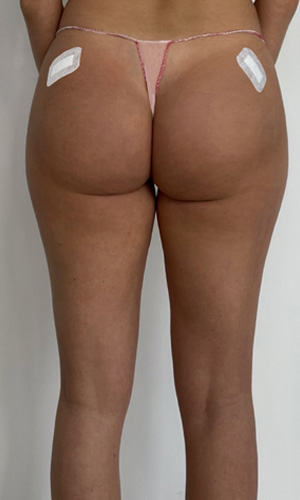
USG AFTER
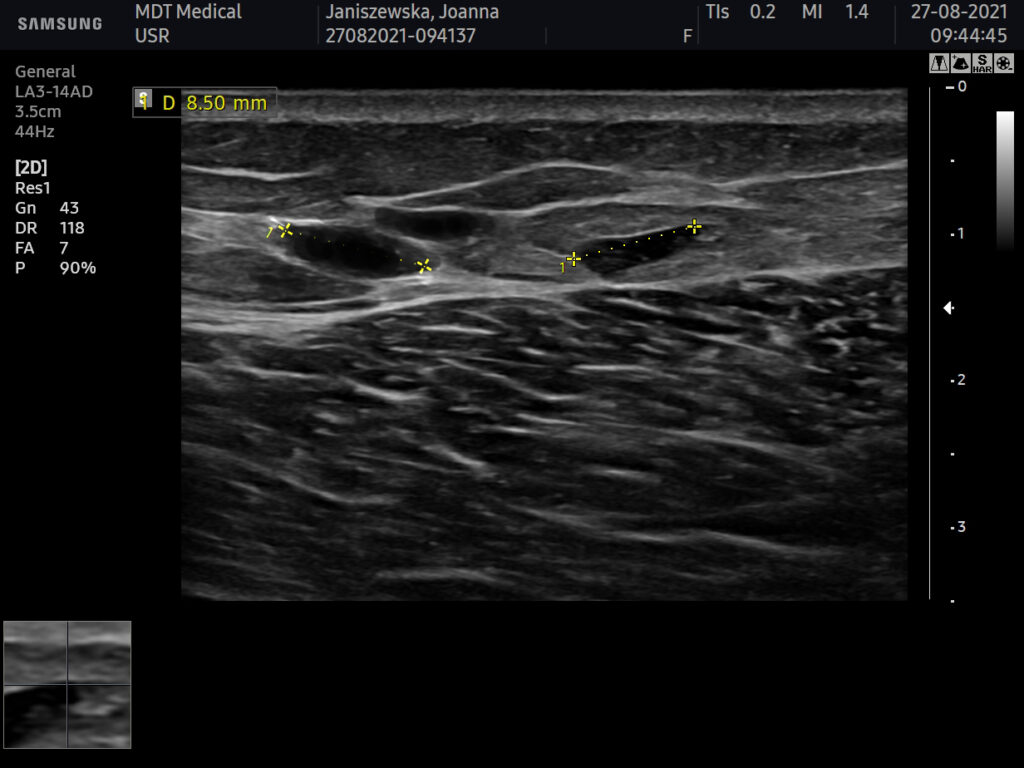
Ultrasound equipment: Samsung HS60, linear transducer 3-14MHz
The ultrasound examination showed an area of the buttocks, 48 hours after injection of the filler.
In the area approx. 15x20cm per buttock, starting slightly below the upper iliac crests to approx. 1/3 of the lower buttock height, fluid foci of decreased echogenicity were seen in the adipose tissue.
FIRST PATIENT
USG BEFORE
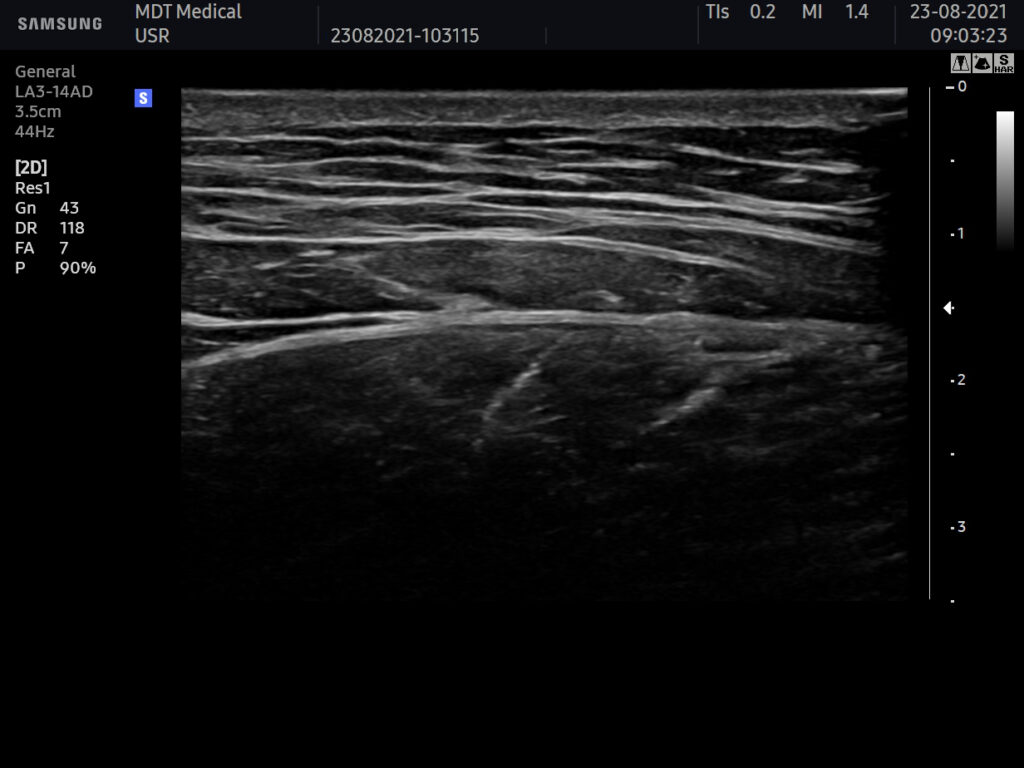
Ultrasound machine: Samsung HS60, linear transducer 3-14MHz
The buttocks area was visualised by ultrasonography. There were no changes visible in the skin and adipose tissue. No pathology within the gluteal muscles.
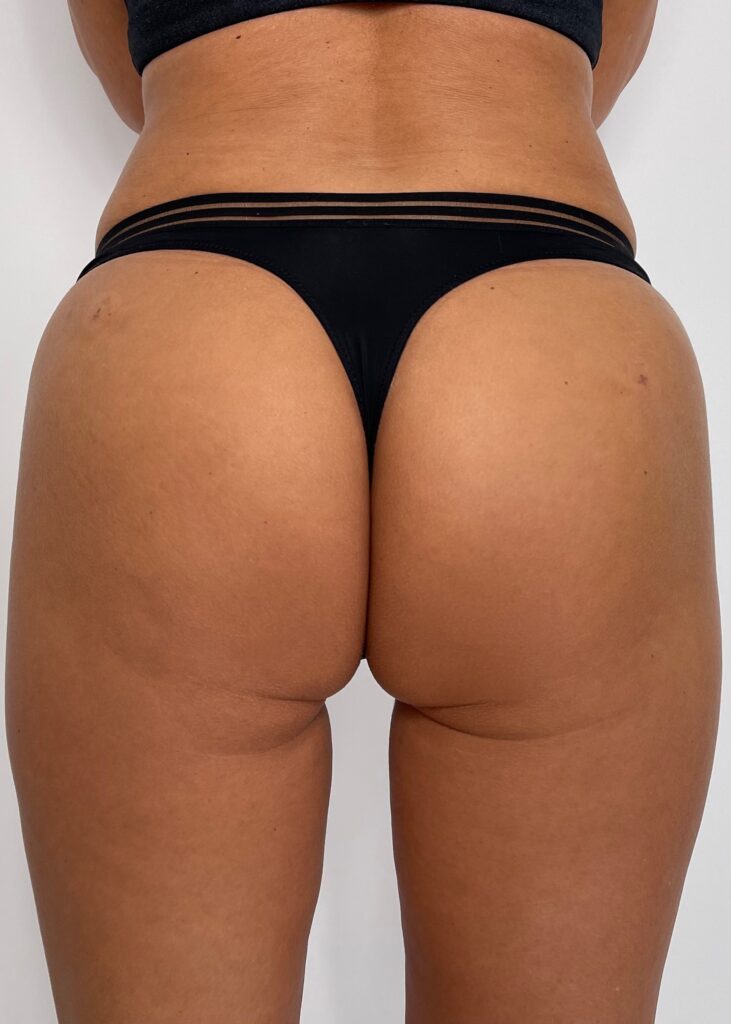
USG AFTER
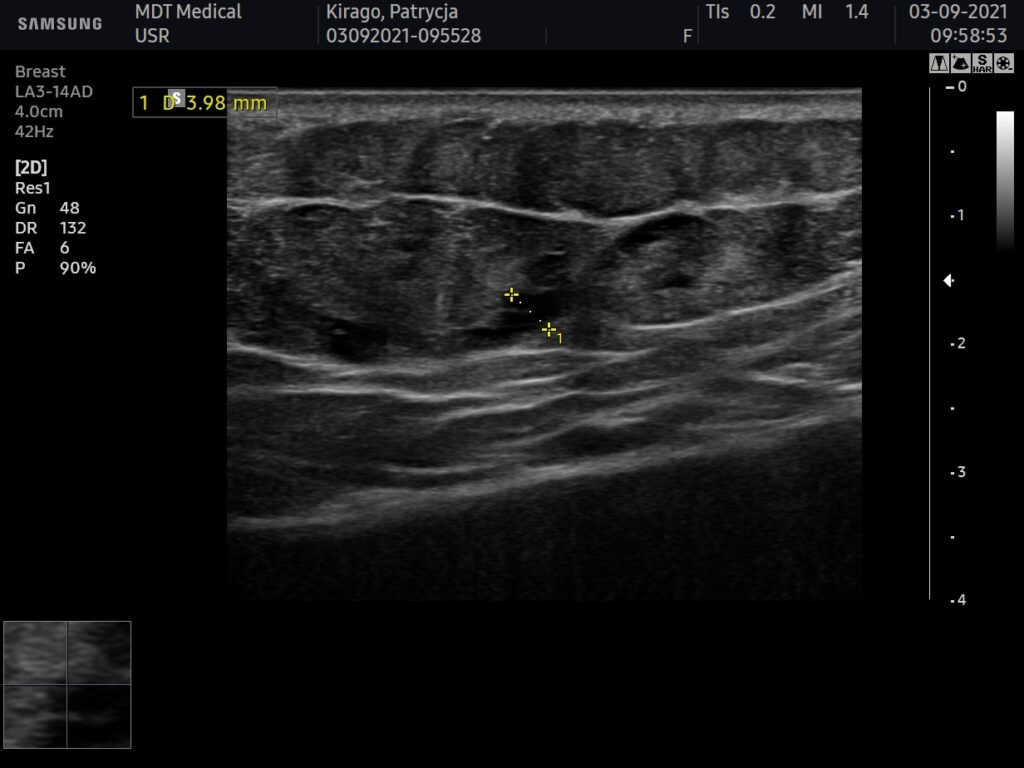
Ultrasound machine: Samsung HS60, linear head 3-14MHz
The ultrasound examination showed the area of the buttocks, after the injection of fillers;
Within the fatty tissue of both buttocks, fluid foci are present in an area of approx. 11×20 cm per buttock, beginning at the height of the visible injection sites on the skin to about 1/3 of the lower buttock height,
They are located between the lobules of adipose tissue, usually about 3mm thick. In transverse dimension, filler is present in both buttocks from about 3cm from the gluteal crevice to the lateral surface near the ileum.
No features suggestive of subcutaneous tissue oedema, no features of abscess on examination.
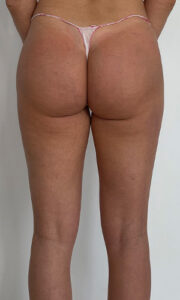
USG AFTER 3 MONTHS
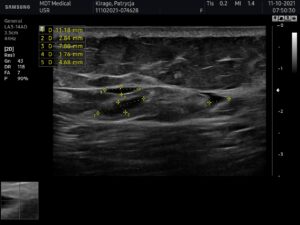
Ultrasound machine: Samsung HS60, linear transducer 3-14MHz
The ultrasound examination showed the buttocks area, just over 3 months after filler injection;
In the fatty tissue of both buttocks, at the level of the greater ileum, in the 1/3 lateral part of the buttocks, fluid collections (up to 11x3mm) were visible. Smaller number of free fluid reservoirs compared to the previous examination (then two days after administration);
Around the reservoirs a slight reaction of the adipose tissue is visible increased
Around the reservoirs a slight reaction of adipose tissue can be seen (increased echogenicity), at a thickness of about 3mm.
No features of abscesses at the time of examination.
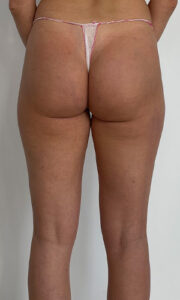
USG AFTER 6 MONTHS
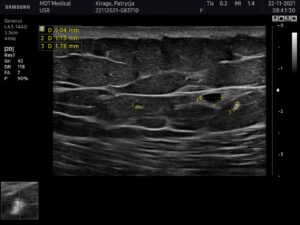
USG apparatus: Samsung HS60, linear transducer 3-14MHz
The ultrasound examination showed the region of the buttocks, 6 months after the injection of the filler;
In the fatty tissue of both buttocks slight fluid collections were seen.
No abscesses at the time of examination.
